Expertise
Cardiac contractile function cardiaque at PhyMedExp
Electrocardiogram
The PhyMedExp laboratory has the expertise and equipment necessary for electrical cardiac functional exploration in small animals (rat mice) either by telemetry in awake rodents or with surface electrodes in anesthetized animals.
Voir section electrophysiologie pour plus de détails
Echocardiography-Doppler
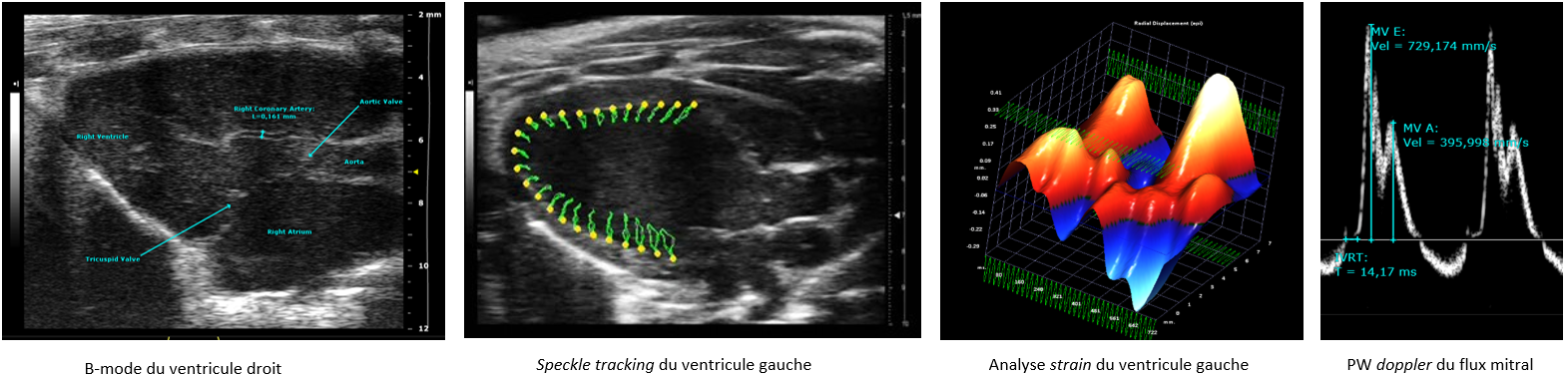
The IPAM/PhyMedExp platform offers high-resolution ultrasound imaging services with a Vevo3100 ultrasound scanner (Visualsonics) equipped with 2 very high frequency probes (MX250: 21MHz, MX550D: 40MHz) specifically adapted to small animal imaging. An imaging station, allows 3D positioning adjustments (micrometric displacements), associated with a guided injection device, favors the realization of high precision imaging protocol.
This ultrasound system allows, in a non-invasive way and in real time, to image cardiac function in vivo in small anesthetized animals (mice, rats) with excellent spatial and temporal resolution. It allows anatomical characterization (eg wall thickness, diameters and volume of the heart chambers) as well as a functional and hemodynamic evaluation of the tissues (eg vascular flows, tissue perfusion, myocardial contractility).
This system is dedicated to the evaluation of left and/or right ventricular function (speckle-tracking, 4D imaging, systolic and diastolic functions)
Contraction of intact isolated cardiac muscles
Analysis of the excitation-contraction coupling of isolated cardiac muscles (papillaries or trabeculae). Muscles are dissected from isolated hearts (mouse, rat, rabbit, guinea pig) and attached to a high-speed motor and force transducer. A dynamic iterative computer algorithm also enables the measurement of simulated pressure-volume relationships. Intracellular Ca2+ can be measured simultaneously by fluorescence (Indo-1) or action potential with an electrode.
Parameters measured: Contraction force, force-sarcomere length relationship, contraction kinetics, intracellular calcium dynamics, action potential
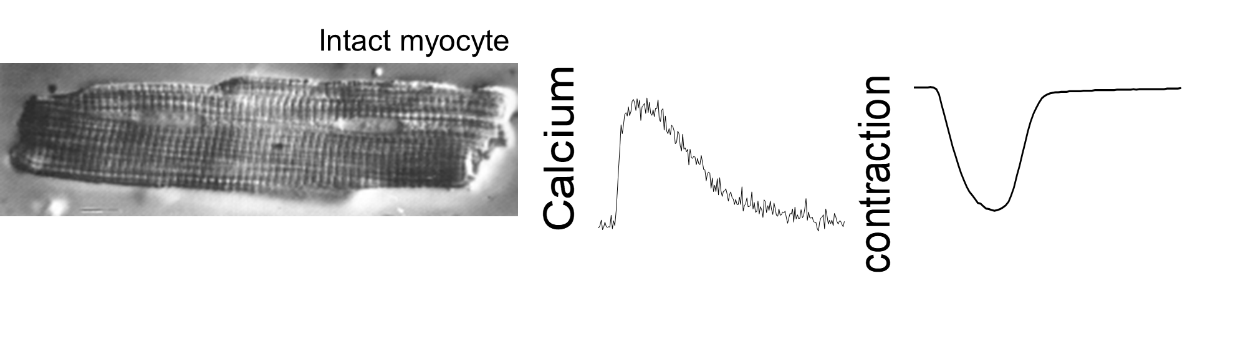
Contraction of free intact cardiac cells: isotonic contraction
Analysis of the excitation-contraction coupling of cardiac cells (mouse, rat, guinea pig). We have two ionoptix systems to simultaneously measure the contraction of isolated cardiac cells (shortening of sarcomeres) and calcium movements by fluorescence imaging. The unit has a fluorescence system for mono-excitation / double emission ratiometric probe (Indo1) and a fluorescence system for double-excitation / mono emission ratiometric probe (Fura, HyperSwitch light source). Other possible fluorescent probes.

Parameters measured: diastolic and systolic values, amplitude and duration of contraction/calcium transient, time of contraction (speed of contraction) and time of relaxation (speed of relaxation).
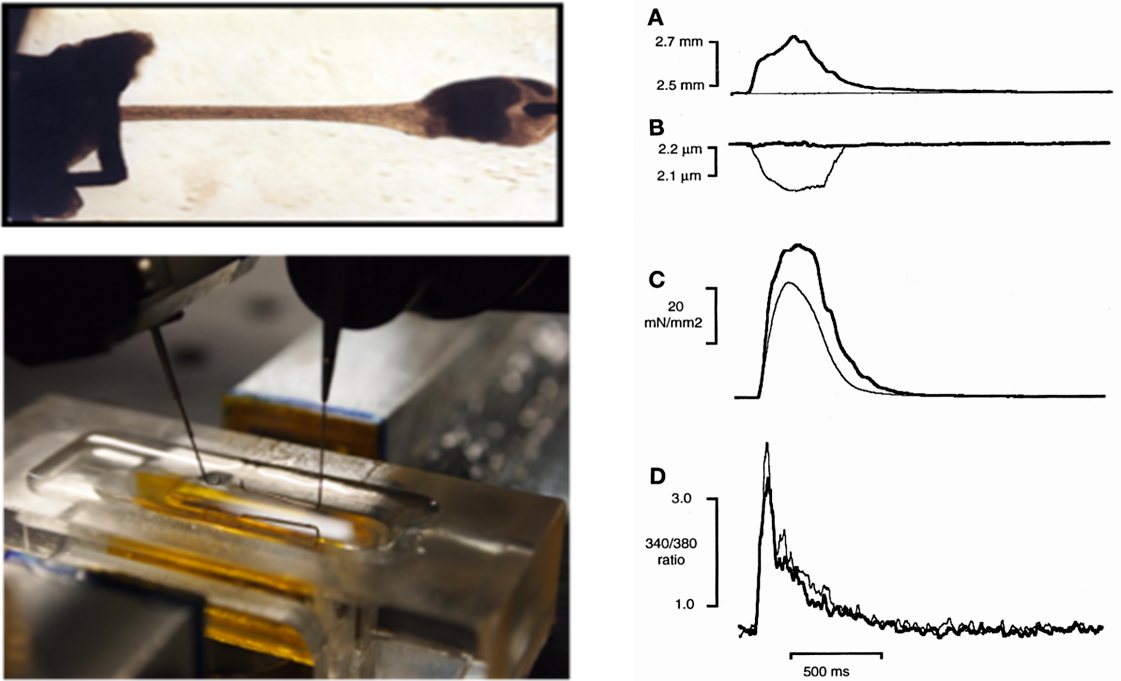
 Contraction of attached intact heart cells: auxotonic contraction
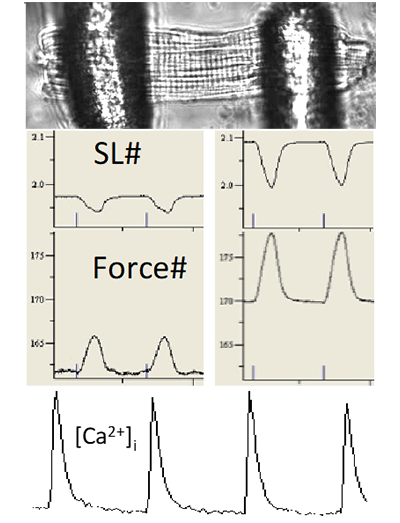
Contraction of attached intact heart cells: auxotonic contraction
Enzymatically isolated myocytes (mouse, rat, rabbit, guinea pig) are attached to glass tips using Myotak glue. The force of contraction is measured either directly by a force sensor or by measuring the calibrated deviation of the tips. The tips are mounted on piezo-mounted micro-manipulators that allow dynamic changes in sarcomere length. Intracellular Ca2+ is measured simultaneously by Indo-1 fluorescence. A dynamic iterative computer algorithm additionally allows the measurement of simulated pressure-volume relationships.
Parameters measured: Contraction force, force-sarcomere length relationship, contraction kinetics, intracellular calcium dynamics.
Analysis of myofilament properties
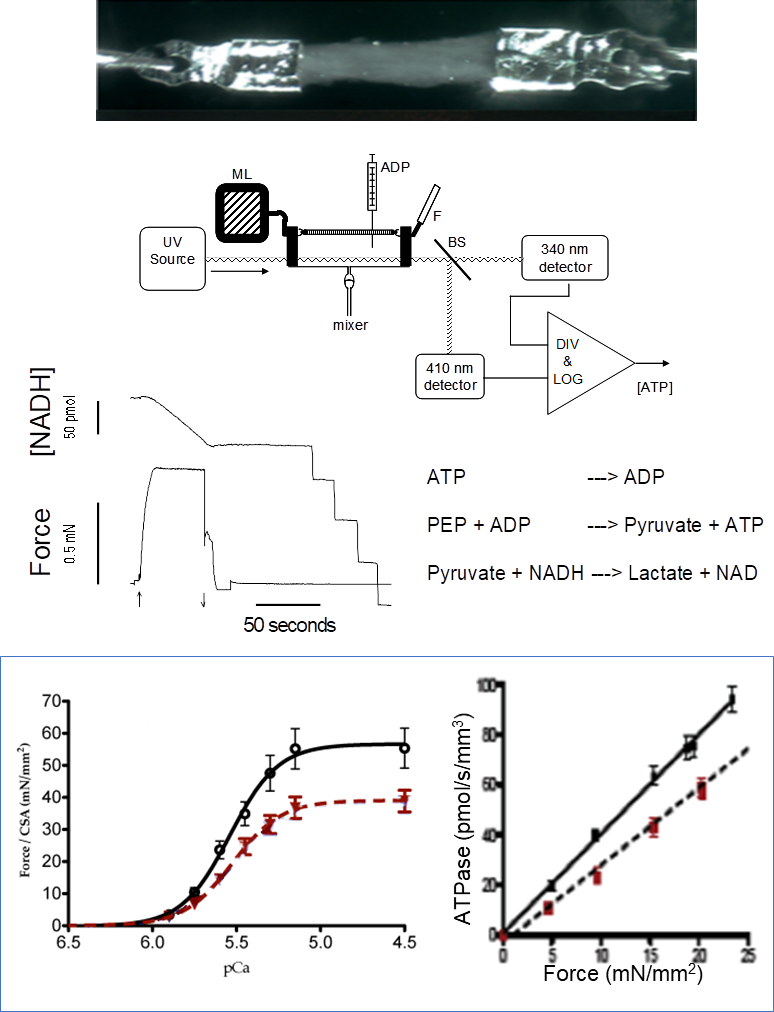
Ca2+ dependency of force development and ATP consumption rate of actin-myosin bridges in isolated permeabilized cardiac and skeletal muscle
ulticellular tissues are prepared from freshly isolated muscles or previously frozen specimens (skeletal or cardiac muscle; mouse, rat, guinea pig, human). The muscles are attached to aluminum T-clips (image shows a human left ventricle trabeculae). The permeabilized muscle is exposed to a series of solutions with variable [Ca2+]. ATPase activity of actin-myosin bridges is measured by a coupled enzymatic reaction (as shown) where the rate of NADH oxidation is stoichiometrically coupled to the release of ADP from the bridges.
Parameters measured: Force-[Ca2+] relationships, ATPase-Force relationship, Ca2+ sensitivity of Force and ATPase activity, tension-cost, bridge kinetics. The figure illustrates the drop in strength and the cost of tension in the myocardium of guinea pigs in heart failure (red) compared to a control heart (black).
Analysis of myofilament properties : contraction of permeabilized cardiac cells
Specific analysis of the properties of the contractile machinery (myofilaments) on permeabilized cardiac cells (mouse, rat, guinea pig, pig, dog, human). Cardiac cells are isolated either by enzymatic digestion of a “fresh” heart or by a mixing technique from a frozen heart. The cells are then permeabilized with a detergent. The cell is glued to metal spikes, one of which is connected to a force sensor and the other to a motor to make rapid changes in length.
Parameters measured: cell rigidity, calcium sensitivity of force, maximum active tension, kinetics of formation of actin-myosin bridges.
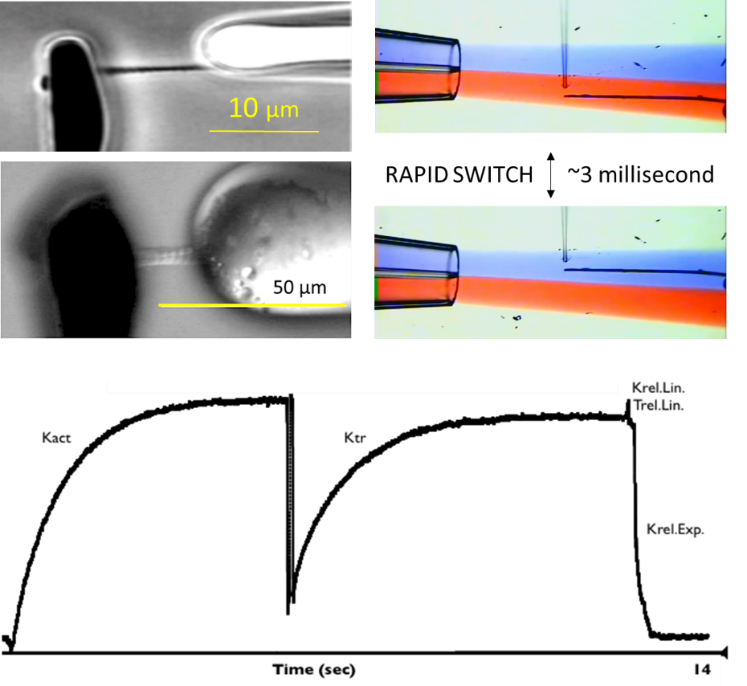
Activation/relaxation dynamics of myofibrils isolated from striated muscle
During a contraction, the cardiac or skeletal contractile apparatus is temporarily activated by a rapid rise and then a rapid fall in intracellular Ca2+. The time course of contraction is determined by the temporal properties of the Ca2+ activation signal and the temporal dynamic response of the contractile protein machinery. The unique myofibril technique allows the biophysical assessment of this vital property. Isolated myofibrils are obtained by homogenization of frozen skeletal muscle (upper example: rabbit psoas) or cardiac muscle (lower example: human left ventricle). Typical dimensions: 30 to 100 µm in length and 3-7 µm in diameter. The myofibril is attached to glass micro-tools that serve as a rapid length controller and force sensor. Myofibrils are perfused through a system capable of changing the composition of the perfusion solution in ~3 milliseconds. Rapid release-stretch maneuver during full activation allows assessment of bridge cycling kinetics (Ktr).
Parameters measured: activation rate (Kact), force recovery rate (Ktr), linear biphasic (Krel.Lin and Trel.Lin) and subsequent exponential relaxation rate (Krel.Exp), peak force development, sensitivity to Ca2+. Additionally, the dynamic response of the myofilament to any rapid change in substrate (e.g. phosphate) can be measured